Pfizer / Hospira
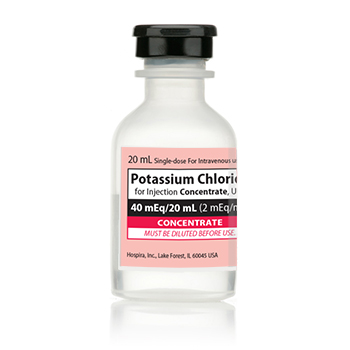
POTASSIUM CHLORIDE FOR INJECTION CONCENTRATE, USP, SDV, 2MEQ/ML (40MEQ/20ML), 20ML NDC # 00409-6653-05 NON-RETURNABLE
SKU030827238000
NDC00409-6653-05
Manufacturer Number00409665305
NDC Number 00409-6653-05 / 00409665305
Manufacturer: Hospira
Application Replacement Preparation
Strength: 2 mEq/mL
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intravenous
Volume 20 mL
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
EACH VIAL OR TRAY 25
NON-RETURNABLE