Pfizer / Hospira
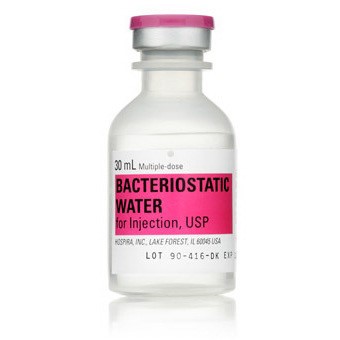
BACTERIOSTATIC WATER 30ML FOR INJ, USP, MDV NDC 00409397703 NON-RETURNABLE 25/PACK
SKU029450054500
NDC00409-3977-03
Manufacturer Number00409397703
Application Diluent
Container Type Multiple Dose Vial
Generic Drug Name Bacteriostatic Water for Injection
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 30 mL, Pack of 25 VIALS
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.
“Preservative Information” indicates that this product does not contain ingredients identified as a preservative, as defined by the USP.