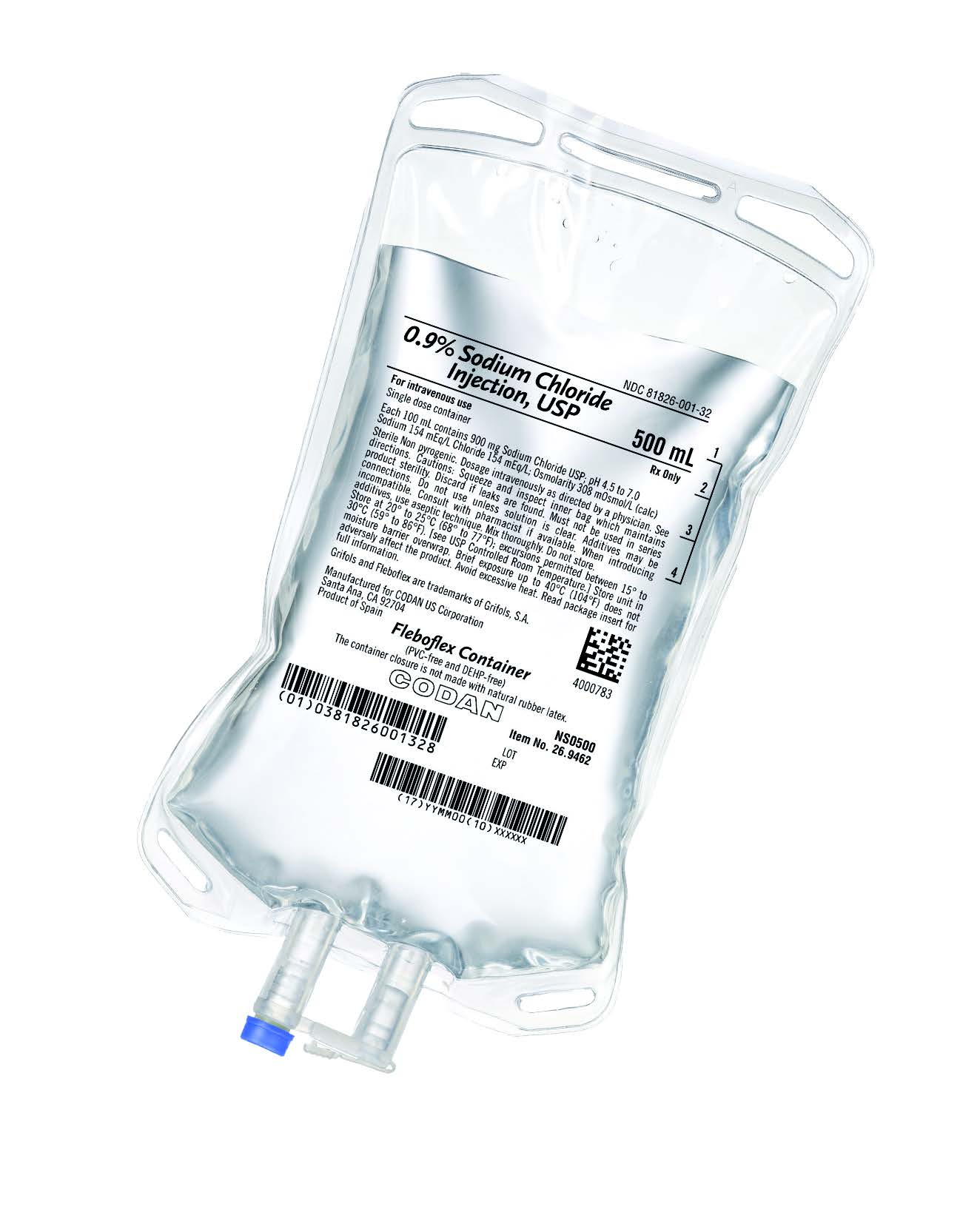
Codan 0.9% Sodium Chloride Injection USP, 500mL Case 20,
No Free Shipping
SKU036159124648
NDC6521947220
Manufacturer NumberFKNS0500US
Generic Drug Name Sodium Chloride, Preservative Free
CODAN/FRESENIUS 500ML, 0.9% SODIUM CHLORIDE INJ, USP, FREEFLEX BAG, 20/CS
NDC # 65219-472-20 / 6521947220
Manufacturer # FKNS0500US
Manufacturer CODAN
Container Type Flexible Bag – 20 Bags Per Case
Dosage Form IV Solution
Type Intravenous
Size Volume 500mL
0.9% Sodium Chloride Injection, USP contains 9 g/L Sodium Chloride, USP (NaCl)
with an osmolarity of 308 mOsmol/L (calc). It contains 154 mEq/L sodium and 154
mEq/L chloride.
Not manufactured with latex, PVC or DEHP.
Non-Returnable.