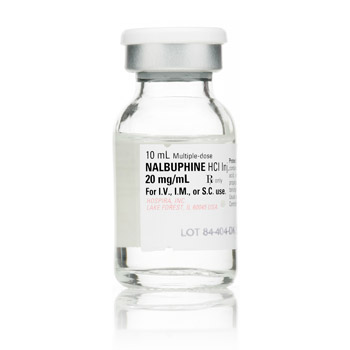
Nalbuphine Hydrochloride Injection 20 mg/mL 200mg/10mL
Manufacturer Hospira/Pfizer
Strength: 20 mg / mL
10mL Vials
Flat of 25 Vials
DEA Required : Opiate Partial Agonist
Product Is NON-RETURNABLE.
Note Dating: Expiration date maybe within 90 days. Please call us to confirm expiration date. 800-69-MERIT.
Storage Requirements USP Controlled Room Temperature
NOTE:
1. We must have your current DEA license on file. Please email us the license at solutions@meritpharm.com or fax to (323) 227-4833.
2. The product may only be shipped to the address on your DEA license.
TO BE IN COMPLIANCE WITH DEA/STATE REQUIREMENTS, ORDERS ARE SUBJECT TO CANCELLATION.
ALL DEA REQUIREMENTS MUST BE MET BEFORE AN ORDER IS PROCESSED. IT IS YOUR RESPONSIBILIY TO MEET THE REQUIREMENTS.