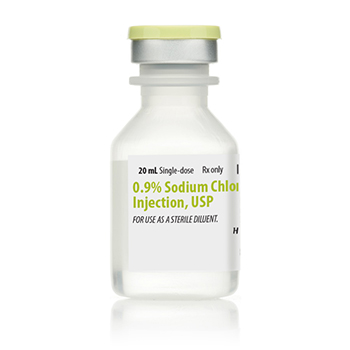
0.9% SODIUM CHLORIDE 20ML INJECTION, USP, SDV NDC 00409488820 NON-RETURNABLE
SKU0320043265000
NDC00409-4888-20
Manufacturer Number00409488820
Manufacturer: Hospira
Application Diluent
Strength: 09%
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 20 mL. Pack of 25 vials. Quantity: 25.
Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.