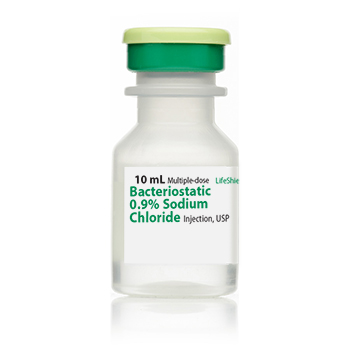
Bacteriostatic 0.9% Sodium Chloride Injection, USP Pack of 25 x 10mL vials NDC# 00409-1966-12
SKU030318259437
SOLD AS A PACK OF 25 x 10ml Vials
NDC Number 00409-1966-12 / 00409196612
Manufacturer: Hospira
Application Diluent
Strength: 0.9% Bacteriostatic Sodium Chloride
Single Dose Vial
Storage Requirements USP Controlled Room Temperature
Type Intramuscular, Intravenous, or Subcutaneous
Volume 10 mL
Latex Free: Latex Information indicates that natural rubber latex has not been used in the manufacture of this device or drug container closure system.